Table Of Content
We talk to a familiar guest and medical device expert Michael Drues Ph.D., president of Vascular Sciences. Michael works for and consults with medical device companies located all over the world. He also works with the US FDA, Canada Health, and other regulatory and government agencies in the US, Canada, Europe and elsewhere in the world.
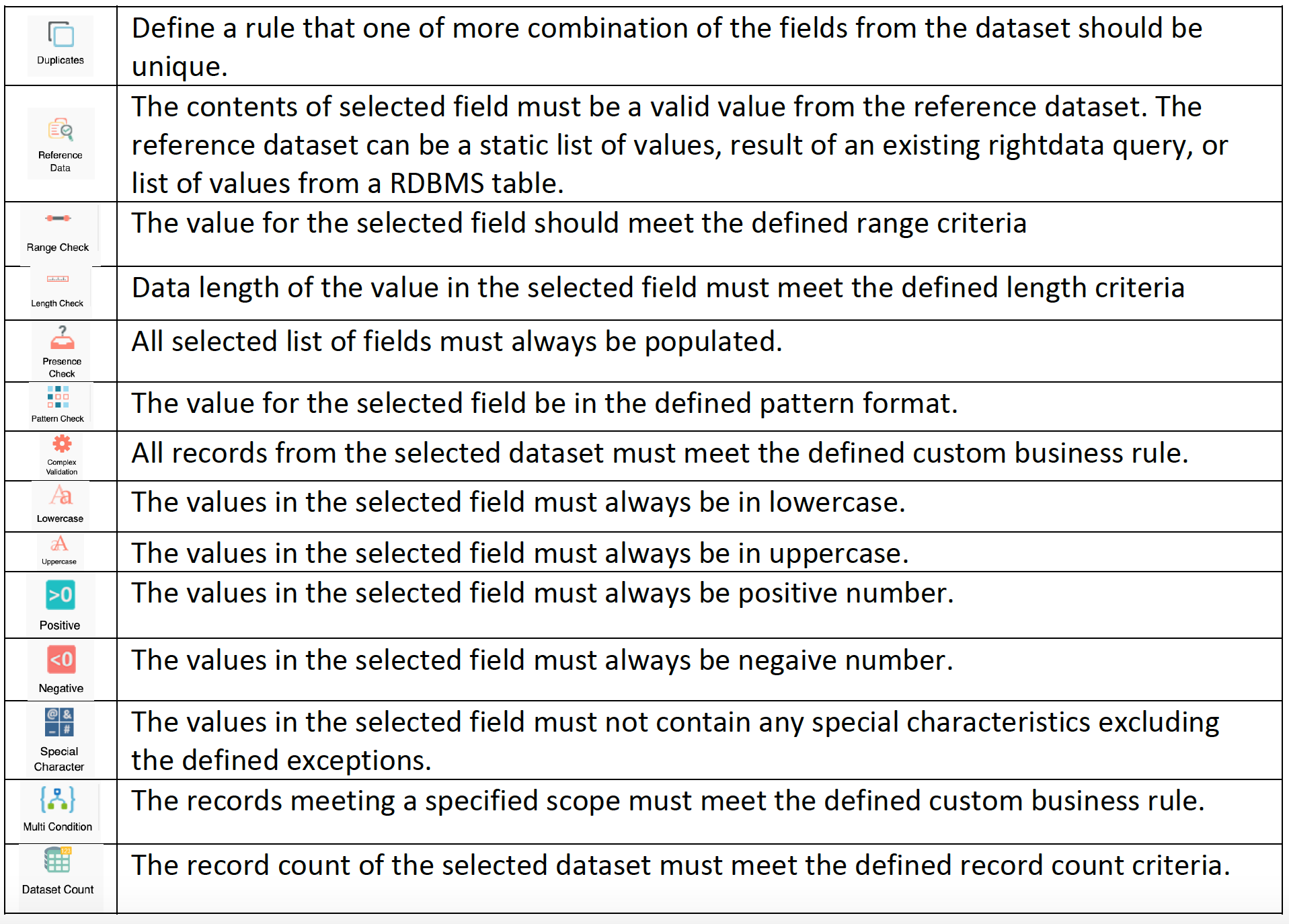
Advantages of design validation and verification
Contact us today if you would like to automate your manufacturing process. The objective of running an EVT test is to test the functionality of the components you want to include in your product by carrying independent pre-studies of each part. During the above tests, the team will identify any issues that need rectifying before designing the final product. Product testing is important because it’s the best way to see how your ideas, prototypes, and products perform in real environments with real users. You’ll need to run different types of tests depending on what stage you’re on in the product design process and what your goals are.
With his Heisman returned, Reggie Bush vows to continue NCAA fight: ‘I never cheated’
These are different activities which are performed at every stage of development process. Through objective evidence, this process will consistently examine that the product meets the predefined requirements. This process involves testing activity, inspection and analysis, and so on. Elena is a T-shaped UX Researcher with a varied cross-industry marketing background. She holds a BA in English as SLA, a Master of Science in Management and a professional UX Design Diploma. For design validation, A/B testing is usually conducted on prototypes to spot critical issues before jumping into the development phase.
Validating a product with the wrong user
It takes much less maintenance effort to maintain traceability as your requirements, designs, and tests evolve than it does to patch critical holes in design and development at the 11th hour. This effort can also help you identify how much work is left, where you might need to add development or test staff, or when you should re-evaluate delivery schedules. Getting your team on the same page is critical to successful design validation & verification. Using the same terms eliminates confusion for team members (not just new members — veterans, too). See the glossary of terms and common acronyms below to help develop your foundation of terminology. It’s impossible to successfully execute a plan without the right team.
Difference between Design Verification and Validation
Design and validation of a microalgae biorefinery using machine learning-assisted modeling of hydrothermal liquefaction - ScienceDirect.com
Design and validation of a microalgae biorefinery using machine learning-assisted modeling of hydrothermal liquefaction.
Posted: Tue, 15 Aug 2023 19:41:05 GMT [source]
Analyzing and validating a product design through the lens of design heuristics is quite common. Experienced or not, though, a designer should have these heuristics top of mind. To help you do that we’ve prepared a checklist and a template for Nielsen Heuristic Analysis. Ideally, you’d need to print these templates and have an analysis session on your app.
It’s easy to collect the data and never do anything with it—but that’s a waste of resources and valuable user insights. It’s a common practice to outsource verification testing to an ISO certified lab. These labs manage their tools and equipment in accordance with international standards, ensuring your verification results are legitimate.
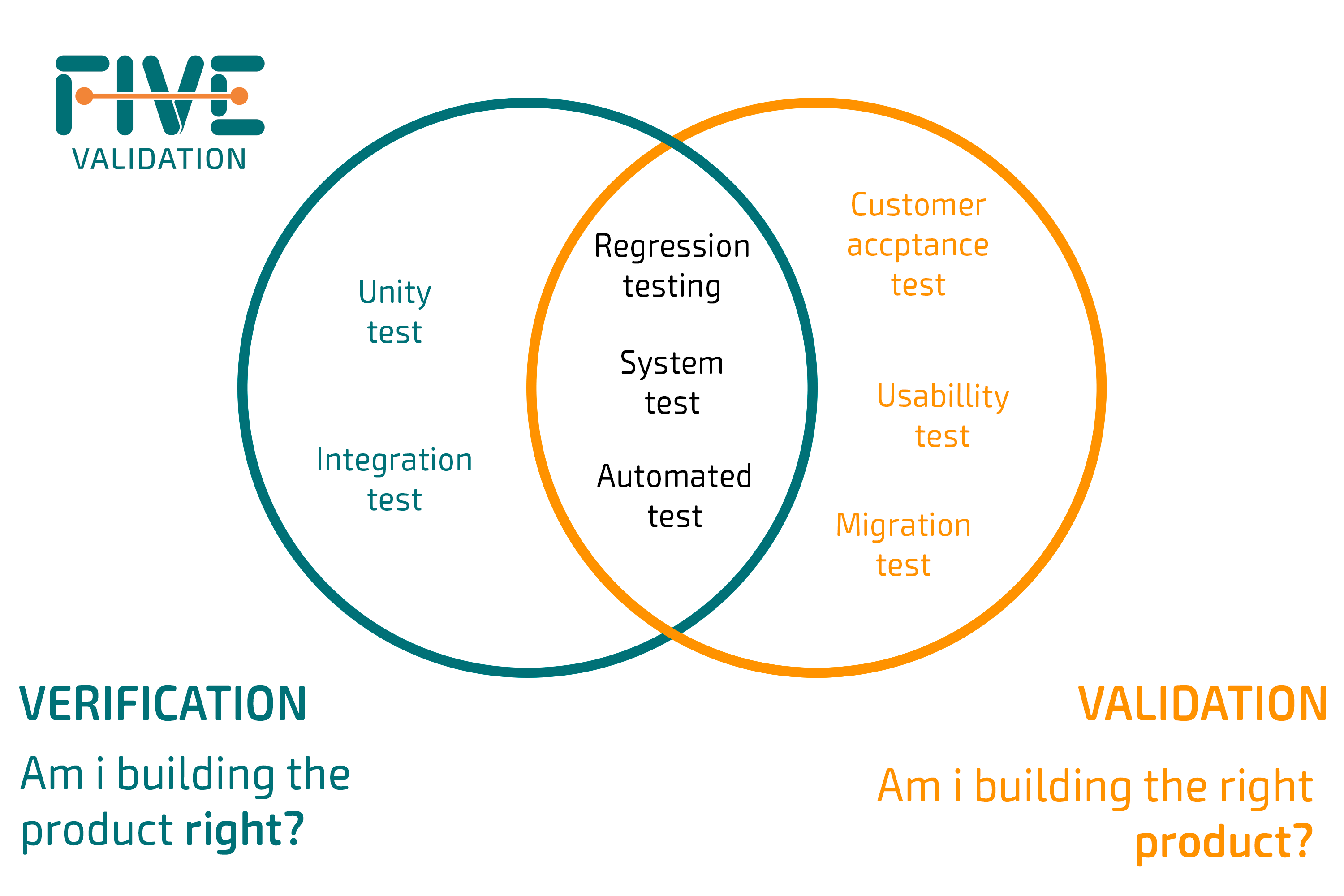
For a good visual representation of this dynamic, simply look at the FDA’s design controls waterfall diagram. Traceability – The ability to ensure that requirements outlined in the specifications have been tested. It’s conducted using the methodology of choice (Scrum, Waterfall, hybrid, etc.).
How Do EVT, DVT, and PVT Impact the Manufacturing Process?
This helps to assess the device’s performance in actual clinical settings and verify its effectiveness and safety under various conditions. The data collected during clinical validation is crucial in supporting claims about the device’s intended use, therapeutic benefits, and risk mitigation measures. By adhering to these guidelines and documenting the validation processes accordingly, manufacturers can ensure that their devices undergo rigorous and comprehensive testing. This approach helps to demonstrate compliance with regulatory standards and fosters confidence in the safety, effectiveness, and reliability of the medical devices.
Angels are swept by the Twins, losing for the ninth time in 10 games
In industries where trust is of utmost importance, such as banking or healthcare, design verification is crucial to ensure that products are released as they are intended to be used. Without conducting design verification, you might give users confusing or frustrating experience that could lead them to question the trustworthiness of your product. You can always leverage previous lessons, experiences, and data, and the types of design changes you’ve made will determine how much verification and validation you’ll need to redo.
This enables us to focus on designing experiences that solve the right problems. Verifying designs then ensures that those experiences align with user needs and solve the problem effectively. If you don’t have plans in place for verification and validation, you’ll end up wasting an enormous amount of time on these steps, and you may miss key activities that could hold up your regulatory timeline. Design verification and validation are crucial to releasing a final product that meets stakeholder and customer needs. Mistakes or oversights can cause serious problems with the final design. Greg Jung has more than 25 years of experience designing medical equipment and electro-mechanical products for a wide variety of industries.
This phase creates one last chance for your manufacturing team to tweak the production process. They can determine whether mass production is ready by stage-gating it into different red, orange, and green stages. The red in this case means that the team needs to perform some significant tweaking, orange means some minor changes, and green signifies that mass production is ready. At this stage, your engineering team incorporates and optimizes the crucial functional scope of the product.
An example of design validation for a mobile app would be conducting user testing and interviewing subjects to get their feedback. This is a great way to see how users experience the app and reveal any issues. Although it’s easy for professionals to incorrectly interchange the terms, "design verification" and "design validation" have very different meanings. Validation – Establishing by objective evidence that device specifications conform with user needs and intended use(s).
To validate this, you would engage a qualified outside source such as a surgeon and have them evaluate your product to ensure it meets its intended use. They may validate that yes, you have met the intended user requirement, or no, you have not. In the FDA’s waterfall diagram of design controls, you can see that the process begins with determining the user needs for your device. That means that gauging metrics about design changes is how you can estimate if you made a good design decision. Additionally, you’d have to make as few changes as possible to estimate each decision separately, or else you won’t know what works and what doesn’t.
No comments:
Post a Comment